On November 17th, according to the latest research published online by Science, Nankai University has made a significant breakthrough in the field of inorganic synthesis and coordination chemistry. The research, titled An all-metal fullerene: [K@Au12Sb20]5-, reports the synthesis and bonding mechanism of the first-ever all-metal fullerene [K@Au12Sb20]5- (see Figures 1-2). This study showcases a novel compound synthesis technique and precise manipulation of metal-metal bonds' structural chemistry applications, offering fresh perspectives for the creation of new materials. This groundbreaking achievement underscores Nankai University's crucial position in the fields of synthetic chemistry and main group metal element coordination chemistry.
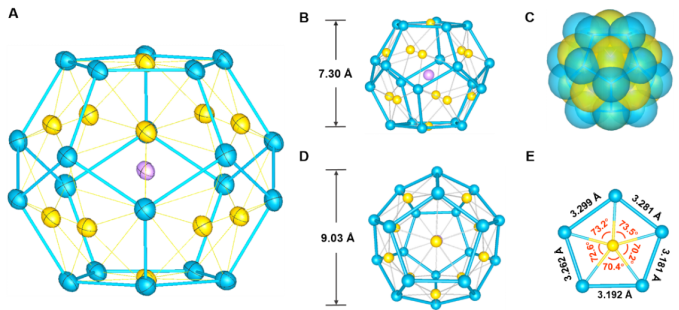
Fig. 1. Molecular structure of the [K@Au12Sb20]5- cluster. Color scheme: K (cyan), Au (gold), Sb (blue).
Since the year 2000, the Nobel Prize in Chemistry has been awarded seven times to scientists in the field of synthetic chemistry. This year, it was presented to three scientists who have made original contributions to the discovery and synthesis of quantum dots. Looking back to 1985 when Smalley and others first discovered the spherical carbon clusters composed of 60 carbon atoms, known as C60, or fullerene, they were awarded the Nobel Prize in Chemistry in 1996 for this groundbreaking discovery. Fullerene has garnered significant attention ever since due to its highly symmetrical structure, distinctive physical properties, and a wide array of chemical reaction characteristics. This has led to continuous exploration of its applications in various fields. Fullerene's bonding characteristics have also gradually extended to the field of inorganic synthesis chemistry. Theoretically, inorganic fullerenes are predicted to exhibit exceptional stability and reactivity, sparking great interest among scientists. However, their synthesis still poses significant challenges.

Fig. 2. Bonding patterns and canonical molecular orbitals for [K@Au12Sb20]5-
Led by Professor Zhong-Ming Sun at Nankai University, a research team has successfully developed a new synthesis method that combines high-temperature solid-phase synthesis with organometallic chemistry to prepare the all-metal fullerene [K@Au12Sb20]5-. This compound exhibits a structure close to an Archimedean dodecahedron, with each face composed of an antimony pentagon containing a single gold atom, with an inner diameter of approximately 0.90 nanometers, slightly larger than the diameter of a C60 molecule (0.71 nanometers). What's astonishing is that within this relatively large cluster cavity, only one potassium cation is embedded, and the cluster as a whole does not require the protection of organic ligands while still maintaining excellent chemical stability. This makes it the closest pure inorganic compound to a fullerene in terms of its coordination environment. The stability of this exposed heavy metal spherical cluster can be attributed to the central potassium cation playing a role in template support, and the unique metal-metal bonds between gold and antimony play a crucial role in maintaining the overall structural integrity (see Figure 2).
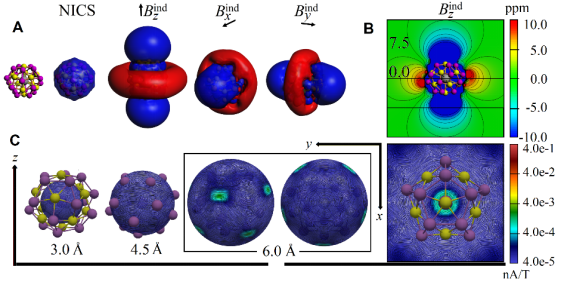
Fig. 3. Electronic structure for [K@Au12Sb20]5-
Theoretical calculations reveal that one of the most significant features of this molecule is its three-dimensional aromatic electron structure, leading to the formation of a layer of delocalized π electron cloud on the cluster's surface. This imparts unique physicochemical properties to the all-metal fullerene compound (see Figure 3). This discovery holds the potential for significant contributions in fields such as optoelectronic materials or room-temperature catalysis, demonstrating broad application prospects.
Graduate student Yu-He Xu from the School of Materials Science and Engineering at Nankai University is the first author of this research paper, with Professor Zhong-Ming Sun serving as the corresponding author. This work received theoretical computational support from Dr. Wen-Juan Tian at Shanxi University, Professor Muñoz-Castro from the University San Sebastián in Chile, and Professor Frenking from the University of Marburg in Germany. Additionally, this research was financially supported by the National Natural Science Foundation of China, the Tianjin Municipal Science and Technology Commission, and Nankai University.
