
Tingting Yan, Weili Dai*, Guangjun Wu, Swen Lang, Michael Hunger, Naijia Guan, Landong Li*
ACS Catal, 2018, 8: 2760-2773 [ pdf ]
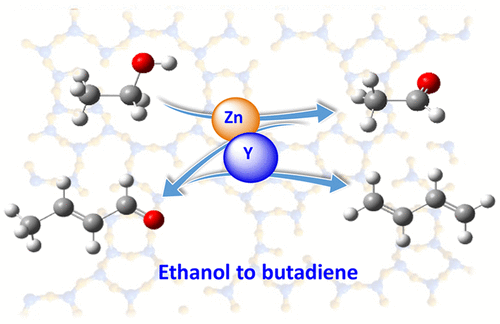
Bifunctional Zn-Y/Beta catalyst was applied in the reaction mechanism study of the ethanol to butadiene conversion, to clarify the roles of Zn and Y functional sites in each individual reaction step. According to the results of several complementary methods, i.e. ethanol temperature-programmed desorption reaction (TPD), temperature- programmed surface reaction (TPSR), and in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), the reaction network consisting of several key steps, i.e. ethanol dehydrogenation, acetaldehyde aldol condensation, and crotonaldehyde reduction, was elucidated. An enolization mechanism was verified to involve in the coupling step. During this reaction, the Lewis acidic Zn and Y species in [Si]Beta zeolite were both active in the ethanol dehydrogenation, aldol condensation, and Meerwein-Ponndorf-Verley reduction. In this cycle, Zn species exhibited the higher dehydrogenation activity but lower coupling activity than that of Y species. Through the combination of the two species in one catalyst, i.e. Zn-Y/Beta, the synergistic effect of the bifunctional sites could be achieved. Our study provides mechanistic insights into the cascade transformation of ethanol to butadiene and the fundamental guidelines for the rational design of eligible catalysts for the reaction.
