--- 材料科学与工程学院-中文 ---

论文题目:Facile Preparation of Methyl Phenols from Ethanol over Lamellar Ce(OH)SO4·xH2O
论文作者:Jinqiu Guo, Zongjing Feng, Jun Xu, Jie Zhu, Guanghui Zhang, Yaping Du*, Hongbo Zhang, Chunhua Yan
Abstract:
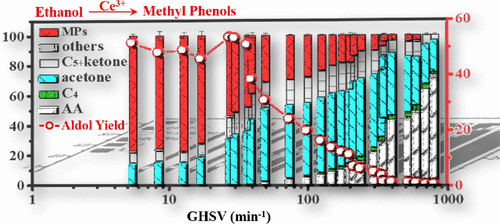
Ethanol transformation with high product selectivity is a great challenge, especially for high weight molecules. Here, we show a combination study of kinetic, thermodynamic, and in situ spectroscopy measurements to probe the selective upgrading of ethanol over lamellar Ce(OH)SO4 center dot xH(2)O catalysts, with 60-70% Ce3+ preserved during the catalysis. High methyl phenols (MPs) selectivity at similar to 80% within condensation products was achieved at similar to 50% condensation yield (3.0 kPa C2H5OH, 15 kPa H-2, Ar balanced, 693 K, 1 atm, gas hourly space velocity (GHSV) similar to 5.4 min(-1)), with acetaldehyde, acetone, 4-heptanone, and 2-pentanone as the key reaction intermediates. Kinetic measurements with the assistance of isotope labeling proved that MPs generated from the kinetically relevant step (KRS) of C-C bond coupling of enolate nucleophilically attacks surface C2H4O following a Langmuir-Hinshelwood model. Low ethanol and water pressures and high acetaldehyde and hydrogen pressures were proved to be favored for MPs generation rather than dehydration, in which hydrogen could reduce the amount of lattice oxygen and facilitate the preparation of MPs while water and ethanol both compete with acetaldehyde for active sites during catalysis. On the basis of in situ X-ray diffraction (XRD), quasi-in situ X-ray photoelectron spectroscopy (XPS), and Raman characterizations, the Ce(OH)SO4 crystal structure was proved to be maintained along with ethanol activation, and the Ce3+-OH Lewis acid-base pair was proved to be the active species for the selective C-C bond coupling. The KRS assumption was also supported by the apparent activation energy assessment within the reaction network on dehydration, dehydrogenation, aldol condensation, and cyclization and a series of negligible kinetic isotope effects (KIEs). This system can be easily extended to some other systems related to C-C bond coupling and is attracting attention on converting oxygenate platform molecules over lanthanide species.
